Joanna Hull’s investigation into nanolimes and the use of consolidents for stone conservation has gained her a First Class Honours BSc from the University of the West of England. Hopefully it will be published in full online later in the year. It raises important issues for the conservation sector, as the brief summary below indicates.
Latest developments in stone consolidation involve the use of nanolime. In some cases it is considered that these ‘innovative nanolime treatments’ constitute a valid alternative to traditional lime treatments. For her BSc dissertation, Joanna Louise Hull considered the feasibility of the use of such materials in conservation practice in relation to a specific set of historical ecclesiastical buildings.
She suggests that there is not now, nor is there likely to be any time soon, sufficient research or literature relating specifically to the philosophical issues involved in the use of consolidents in general and nanolimes in particular or the practical use of these materials in the conservation of external stonework to ecclesiastical buildings.
Her dissertation provokes thought and, until such time that more information is available, offers a detailed insight into the issues to be considered by those faced with a decision to make regarding the conservation of stone within churches and cathedrals (and, indeed, other buildings), so that careful and justified solutions can be reached.
The dissertation concludes that there is an opportunity for nanolimes to offer a feasible conservation method for limestone churches and cathedrals but clearly options for any conservation are always subjective.
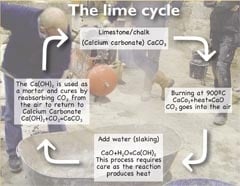
The idea of introducing calcium hydroxide to a decayed calcareous substrate to allow it to carbonate has long been understood. The process of carbonation or, probably more correctly, re-carbonation takes place when hydrated lime (quicklime and water) is exposed to the air and draws carbon dioxide from the atmosphere, replacing the oxide component and turning the lime back to its original state of a limestone.
Calcium hydroxide, referred to in its saturated solution form as limewater, is applied to a stone surface to introduce calcium hydroxide into the stone and consolidate the stone by replacing the lost binding cement within the stone.
The 21st Century has already produced a plethora of research that develops the ideas of stone consolidation in order to improve the effectiveness of consolidents. One of them is nanolime, which uses quicklime in suspension in a solvent rather than water as the consolident.
With the known limitations of previous and existing methods in mind, studies have been carried out that look specifically at the use of nanotechnology as a practical solution to the conservation of stone in heritage structures.
The “effectiveness of inorganic compatible treatments, based on nanosized particles of calcium hydroxide dispersed in alcoholic medium, as consolidents for limestones” that have been “affected by different kinds of decay” (Dei & Salvadori 2006) has been researched extensively.
Brummer et al (2010) discuss the advantages that nanotechnology can offer over more traditional lime techniques, firstly considering the possibility of the nano-particles to penetrate deeply into decayed stone.
By reducing the average size of the consolidating particles to the nanometric scale, say Dei & Salvadori, rather than the previously used micron-sized calcium hydroxide particles, performance of the consolident is increased by achieving a deeper penetration of the dispersion, even when applied on low-porosity limestone that has previously resisted penetration by consolidents (for example Ketton limestone).
That assertion has been question this year (2012), however, by D’Armada & Hirst. They note that: “Lime molecules in limewater are even smaller than nanolime particles.” This is a point hard to find in other literature.
They say nanolimes have better penetration because the calcium hydroxide particles are suspended in alcohol rather than water and alcohol can carry more of them, theoretically carrying greater quantities of lime into the stone.
It is because calcium hydroxide is only in concentrations of up to 1.7g per litre in water that sometimes as many as 40 coats of limewater have been applied to buildings to consolidate the stone.
Alkoxysilanes are used with solvents that allow their reaction rates and depth of penetration into the stone to be controlled. It could be argued that the increased effectiveness of these consolidants is due to the solvents rather than the material itself.
Further, if the solvent evaporates too quickly, penetration will, again, be reduced. This element alone highlights the high level of inconsistencies that can be experienced in the use of stone consolidents.
To add some optimism, it is said that nanolime products, such as the popularly researched CaLoSiL, are of a “high purity, allowing the solvent to evaporate leaving behind no residues” (Brummer et al 2010).
The reactions that occur during consolidation have been said to produce
by-products, such as salts, that have a detrimental effect on the stone. Often trapped behind the newly formed hardened layer of stone, these salts can lead to further decay within the substrate and render the attempt at consolidation useless.
Prof G Ziegenbalg of IBZ Salzchemie GmbH & Co, the manufacturer of CaLoSiL, says: “All CaLoSiL products contain only pure calcium hydroxide… meaning that the reaction with CO2 will produce only CaCO3” (Ziegenbalg 2012, personal communication via email).
While it might be true that the carbonation of the lime would produce no by-products, the same question needs to be asked of the solvent used.
Various solvents can be used with nanolimes – ethanol, n-propanol, iso-propanol – which allow the rate of evaporation to be adjusted as required by the material being treated, taking into account its porosity and considering environmental conditions that affect the rate of evaporation. The sales literature does not communicate the effects of these solvents on people or the environment.
With ever tighter Health & Safety regulations, the VOC (volatile organic compound) emissions produced by alcohols that are harmful to the environment, harmful to health and pose a fire hazard in the early stages (Young et al 1999), could jeopardise the future of stone consolidents using solvents.
On the other hand, Fidler et al (2011) note: “The use of potentially harmful materials has been justified for conservation purposes on the grounds that no substitutes replicate their effectiveness” – possibly a contentious view when the effectiveness of stone consolidating materials is still under consideration.
Although nanolime technology is relatively new, STONECORE (Stone Conservation for the Refurbishment of Buildings, www.stonecore-europe.eu) and other conventions (such as SWAPNET, Stone Weathering & Atmospheric Pollution NETwork) are providing considerable evidence of the development and application of the products through experimentation and case studies. Professor Heather Viles at the University of Oxford is also carrying out research and experimentation in the Back to Nature project.
With such interest, it is possible to understand how nanolime materials could be considered a viable option for conservation.
As Price noted in 1996 of the more traditional method of limewater consolidation, the notion that nothing could be more natural than putting lime into limestone must account for at least some of the popularity of any lime-based product.
However, there is a much more solid basis than this for using it.
It has been proven scientifically that if a saturated solution of calcium hydroxide is allowed to penetrate into limestone, subsequent evaporation of the solution will lead to the deposition of calcium hydroxide within the stone (Price 1996).
The calcium hydroxide will react with carbon dioxide in the air to form calcium carbonate, which then acts as a cementing binder to restore the bonds between the grains and consolidate the stone. That is no surprise because it is the same chemistry that sets lime mortars.
Advice regarding stone consolidation as a conservation method does, however, remain the same now as it has been since the early 1900s: that before any method is chosen all options should be considered.
As Rodrigues noted in 2001, despite a proliferation of studies there has been no serious effort to integrate all the dispersed research results into comprehensive solutions for the practical problems that directly affect conservation practice.
And there remains the question of the place of consolidents within the philosophy of conservation and repair.
English Heritage guidance suggests that in place of renewal or replacement of stone, alternative remedial works should be considered, such as discreet lead flashings or, interestingly, “a surface treatment designed to protect with a sacrificial layer or deeply penetrating consolident” (Ashurst & Ashurst 1988).
However, a principle within conservation philosophy is that any intervention must be reversible so that any new and superior methods of conservation subsequently developed can replace earlier interventions.
The issue faced with stone consolidation is that although it meets the requirement to maintain as much of the historic fabric as possible, it is in no circumstance reversible. Once calcium hydroxide or another material has been introduced to stone its absorption makes its removal impossible.
Of course, cutting out old stone and piecing in new is not reversible, either. But it can be sympathetically and selectively carried out using the same methods to work the stone that were used to create the original masonry.
During the past 30 years there has been an important shift towards minimal intervention. As a technique, even with the conflicting advice and recommendations found within the literature, it is understandable that due to its ability to restore the strength of original fabric there is much interest in consolidation.
But where does it sit with other guiding principles? In terms of authenticity, it could be argued that as a consolidant is intended to be barely visible it is not honestly displaying the fact that a repair or restoration has taken place.
On the other hand, some will see the fact that the intervention is not apparent as an advantage over treatments such as shelter coats that can have striking aesthetic outcomes.
On the other hand, some tests have shown that nanolime can also cause colour changes, producing a white bloom on stones (D’Armada & Hirst, 2012). That might highlight the intervention but is hardly likely to be seen by most as an advantage.
In 2008, the English Heritage document Conservation Principles suggested that it is not only the fabric of a structure that is of value but also its design and function. This allows some flexibility in conservation approaches.
The integrity of a structure applies to design concepts and character, as well as structural systems, materials and the way they are used. This could reinforce the argument that because lime techniques were used in medieval times, and particularly as a matter of course to churches and cathedrals, nanolime treatments are the modern equivalent that is the most honest and authentic course of action to stabilise stone and retard further deterioration.
There appears to be a conflict between the two principles of maximum retention of original fabric and minimal intervention. Although maximum retention might be achieved, minimal intervention is not.
But does the reversibility of an intervention matter if it does not prevent future interventions?
There has been very little consideration in the literature about the effects of a subsequent treatment on an earlier one. Even this year, D’Armada & Hirst have discussed the irreversibility of nanolime consolidation without mentioning the opportunity to re-treat in the future.
This is surprising considering the increased interest in consolidants in modern conservation theory. Research would benefit from investigation into
re-treatability options.
Nanolime consolidents do not stop the stone being porous, nor is it intended that they should result in salts that will block pores, so there should be the potential to use other consolidents in the future.
It is clear stone consolidation is an intricate subject even before adding the complexities involved with the principles of conservation.
In response to the question raised as the title of my dissertation (Can nanolime stone consolidation offer a feasible option for the conservation of limestone ecclesiastical buildings?), it is my opinion that the judgement for each particular element of each building is required and that the answer to the question will most likely always be subjective, as is generally the case with conservation practice.
However, by considering the subjects discussed within my dissertation it may be possible to make a more informed evaluation of the options available in order to reach a sound financial, technical and philosophical verdict.
Joanna Louise Hull